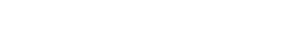Found in the ground is normally covered with a crust of oxides. If you I doubt whether you will be able to remove them, it is better not to do it. In some cases cleaning is a problem that must be given serious attention, as a valuable find very easy to ruin or severely devalued in the process unskilled cleaning, when applied expedited removal methods the oxides. Better consult a professionals who use complex chemicals and even scalpel will remove the slightest traces of corrosion, resulting in the product looks like new, allowing you to see the smallest details of the image for example, on the Roman coin. However, the market of Antiques and collectors not welcome this nature cleaning, preferring to save on the products natural patina.
For those who still wants to try to cleanse products found self, following are some of the techniques and chemicals used for cleaning of various metals and alloys.
Mechanical cleaning
This method involves removal of surface deposits manually. Loose corrosion products can be removed by brushes. For this purpose toothbrushes of varying stiffness. Good results gave a brush to clean fonts writing machines, but now, unfortunately, difficult to find. For large products of iron you can use a wire brush layers of Solid oxides can be pin sharp the tool made out of ordinary sewing needles, the end of which is sharpened like a chisel. The corrosion layer chop piece by piece. The tool should be held upright and watch then, to it does not touch the base metal. The process is slow, but the results can be very good. Very useful during this cleaning to use a desktop magnifier.
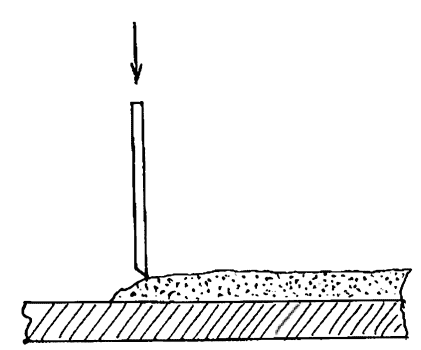
Fig. 60. Mechanical cleaning - spalling oxide-sharpened needle
There are electric tools that accelerates the process of cleansing. It compact drill instead of a drill set of different brushes, as well as vibrating engraving machines that allow for easy chopping with the product solid oxides, using as instruments again or sewing needle a special nozzle.

Fig. 61. Microdrill with nozzles for automatic cleaning
For cleaning large batches of similar products (for example) modern coins, can apply the tumbling reels in which clean items are rotated together with a filler of wood plastic or metal elements in the soap water for a few hours.
Electrolytic cleaning
Equipment for electrolytic cleaning consists of transformer, rectifier and two electrodes - the cathode (-) anode (+). To the anode plate is connected stainless steel, and a cathode - clamp or wire frame that holds a subject that needs to be cleaned from corrosion. Both electrodes are dipped in glass or plastic container into which is poured the electrolyte. Usually it's water with a small amount of salt and a suitable cleaning agent, such as caustic soda or citric acid.
When the current begins a gradual removal of corrosion products. The time from time the current should be switched off and inspect the surface. When the oxides will lose its hardness, the process can be stopped and to conduct further cleaning in a mechanical way.
Electrochemical cleaning
This is another type of electrolytic cleaning in which occur a chemical reaction without the use of an external power source. The cleaned object placed in the container with the solution and add a suitable metal in the form of powder, typically, the zinc in a solution of caustic soda or aluminum in a solution of carbon dioxide sodium.
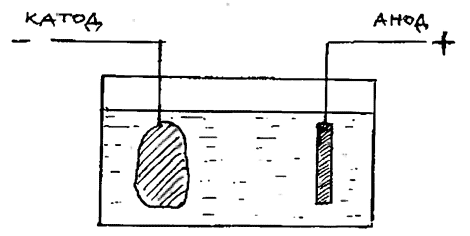
Fig. 62. Electrolytic cleaning
In the chemical reaction of hydrogen, regenerating the oxides on the product.
Chemical cleaning
The product is dipped in a solution of suitable chemicals that may be or acids or alkalis. They dissolve the corrosion products on the product. More than concentrated solutions, the faster the reaction and, accordingly, the process cleaning is more difficult to control.
Iron and its alloys
Iron was used for the manufacture of products exclusively by forging. Casting is not was used due to the fragility of the molded products. Weapons and the tool, in particular axes and knives, were made by forging the floor of the OS variously processed metal While cutting solid core steel was surrounded by a softer iron, whereby the cutting edge in samozatachivanie the process, as the teeth of a beaver, and the tool was always sharp. Unfortunately, iron and steel corrode easily and are the hardest to clean and preserve.
Corrosion of iron is a complex process, which covered with a thick layer of iron oxides or rust. Sometimes this process ends in complete substitution of metal oxides, while the outside retains the product the original shape. This can be checked with a magnet (oxides magnet are attracted).
If you decide to clean the iron object, the method will depend on condition the oxides. Small items such as arrowheads, can have very little the remaining original iron. Therefore, they can be improved by removing file bumpy rust, thereby preserving the shape tip. Larger objects (horseshoes, kernel) can be subjected to more harsh cleaning, for example, to remove rust by cleaving it with a hammer or chisel. Scale with such objects can be easily removed by heating the product up to red heat and subsequent lowering in water or oil.
Mechanical methods of removing rust is more preferable when cleaning iron objects. Some rust is easily removed with a wire brush. More thick layers of oxides you can rip off with a file or abrasive wheel, not forgetting, however, about the initial shape of the object.
When chemical treatment of the oxides can be removed when immersed in solutions mineral and organic acids with the addition of 1-2% of the acid inhibitor corrosion - hexamine, tannin, catechol, hydroquinone, metol. The most active is a solution containing 35% orthophosphoric and 5-10% hydrochloric acid.
After acid cleaning, thoroughly rinse the cleaned surface metal and expose its preservation with the use of a corrosion inhibitor, it is better just benzotriazole.
In some cases it is preferable electrolytic method of removing metal oxides. For this used power supply, 12V (used for charging batteries). The positive wire is connected with the plate of stainless steel (spoon, for example, while the negative wire connect with the purified product. As the electrolyte used in the solution of salt (3 tablespoons to 1 liter of water). The distance between the product and the stainless steel plate is 3 cm as the electrolysis of water becomes brown, and change her every 2-3 hours. After cleaning rinse the item and dry it and cover with wax, varnish or vaseline, to prevent further oxidation.
To clean the surface of the iron from corrosion products are more sophisticated methods to perform at home can only enthusiasts. Such methods, for example, include the recovery in low-temperature gas plasma. This way you can recover items from a fully or almost fully corroded iron, and articles, inlaid with gold or silver.
Furthermore, using the thermal recovery of oxidized iron with of carbon monoxide or hydrogen. Anyway cleaned from corrosion of iron the subject should be treated with some preservative material, as in otherwise it will oxidize again. The first signs of such oxidation is the so-called "exudation", when the surface of the product appear small drops of rusty water, after some time cracks, the metal begins to flake and fall off. Typically, this process is due the presence of chlorides remaining in the corrosion products. To neutralize the chlorides the product is boiled in 5% solution of caustic soda, dissolved in distilled water several times changing the solution. Subsequently, the product is boiled in pure distilled water and dried for several days in drying Cabinet. Finally the product is immersed in acetone, contributing to the destruction of residues of chlorides and after final drying, are immersed for 1 hour in molten wax. The excess wax is removed with a rag and dryer used for drying hair. Finally, the item is coated with any clear varnish.
In restoration practice for ferrous metals are widely used phosphating as one of the reliable ways to protect the metal surface. Depending on the composition of the solution phosphate layer color can vary from colorless to black.
By phosphating you can carry out the conservation of iron objects from significant layers of corrosion products. Formed on the surface of crystalline or amorphous phosphate layers that protects the metal from further corrosion.
Copper and its alloys
Copper was used either in pure form or as alloys. Copper alloy with tin - bronze, an alloy with zinc - brass. Also applied to alloys of lead and other metals, giving the other alloy properties and appearance.
Coins and articles of copper alloys after a long stay in the land, as the rule, oxidized. Oxides of copper are primarily composed of carbonate of copper and have greenish color. However, the corrosion products may be more complex and contain many other elements, oxides which give the different look and may be the reason for their instability.
As a rule, ancient coins and relics with a smooth dense green patina appreciated collectors is much higher than cleaned to shiny metal or the more polished. This means that cleaning in most cases, such objects are not required, except in those cases where they are covered with a thick friable crust of oxides, hiding the details. In this case, it is best to use mechanical cleaning. The removal of corrosion products of the chemical or electrochemical means may cause the removal of patina or its volatility, leading to subsequent corrosion.
Loose corrosion is removed with a dental tool, used in the treatment of the teeth, including bormashenko. It should be borne in mind that articles of copper alloys are often covered with gold or silver that you need to consider when cleaning by mechanical means. Separate solid areas of corrosion usually softened upon exposure of the product in olive oil in 3-5 days. Spot iron corrosion on copper alloys can be dissolved, applying locally a solution of the disodium salt of ethylenediaminetetraacetic acid (Trilon-B).
In some cases, when a thin layer of oxide covers the product with a coating of gold or silver, when mechanical cleaning easy to damage this coating. In this case it is recommended to use a solution of sodium hexametaphosphate. This chemical slowly dissolves the layer of oxides of copper. In processing the product from time time should be removed from the solution and use a soft brush, rinse in water, watching the process. The specified chemical is particularly useful in the event that when the layer of oxides formed on top of the patina. The concentration of the solution should be 5-10%, little heat will accelerate the reaction. Prolonged immersion in this the solution may lead to complete removal of oxides to pure metal. This the solution allows you to remove the calcareous layers, adhering sand and clay. They softened and relatively easily cleaned off with a stiff brush. Processing significantly accelerated by using a hot 20% solution sodium hexametaphosphate (40-50 °C).
Well clean the surface of copper and copper alloys 5-10% solutions of citric and acetic acid, but after treatment in these solutions the products you need rinse thoroughly.
One of the methods cleaning products from copper and copper alloys is boiling them in sunflower or other oil.
Fig. 63. The cleaning results
The oxides thus softened and easily removed with a brush, but the products themselves often acquire black color that is not always desirable.
To clean bronze with gilding applied neutral and alkaline solutions sagatavoj salt. Rochelle salt does not react with the oxides of copper, and removes only salts and their hydrates. In some cases cleaning products can be used electrolytic and electrochemical methods. Electrolytic cleaning is carried out a 5% solution of citric acid at room temperature and current density 3-5 A/dm2. The product is connected to the negative wire as the anode used stainless steel plate. From time to time and the product is removed washed, watching the cleaning. Perhaps when a part of the oxides will be removed, further cleaning should be by mechanical means.
Electrochemical cleaning is applied to the product paste powdered zinc, aluminum or magnesium 10-15% solution of caustic soda. Liberated during the reaction hydrogen contributes to the recovery of salts and copper oxides to the metal.
Bent objects from copper and its alloys can be straightened by heating them in a flame a gas burner. However it is not necessary much to overheat, otherwise the product may undergo significant oxidation and simply burn out.
The appearance of some products can be improved by patina. Patina can be loose or coarse grained. If the color is too light patina or heterogeneous, useful treatment of colored wax. You can use colored cream shoes (green, brown or black). Ordinary beeswax is also used as a protective varnish.
If I had to clean out the product to bare shiny metal, it is possible to improve types it is possible to recover the patina by immersion of the device in the appropriate solutions.
Many products of copper alloys after extracting them from the soil remain stable and does not require special handling for their conservation. Can use a wax or protective coatings for extra protection and improvement appearance.
Sometimes the products are visible separate small areas of green poroshkovaya oxides, which come to the surface from the depth of the product. These oxides due to the presence of chlorides contained in the corrosion products, and which can be activated if the neighborhood is changing. It can also to happen when the object is exposed to chemical or electrochemical treatment. Having arisen, such corrosion may progress and, if she does not pay attention, can lead to complete destruction of the product.
The only way to deal with this disease is the complete removal of the affected plots. This can be achieved by the complete removal of oxides from the product to pure metal or by removing oxides with only the affected areas with using a dental tool. If any traces of such oxides will remain, the process goes on.
For preservation of the product after removal of oxides of copper, it is recommended to handle it in the benzotriazole. Before this product, it should be degreased in alcohol or acetone, after a long time soaking in a solution of caustic soda.
Subsequently, the product is dried and immersed for several days in a 5% alcohol a solution of benzotriazole. After that it is thoroughly dried and removed brush any appeared to precipitate it. Then, the product is applied several layers protective lacquer.
Silver and its alloys
The silver is usually alloyed with other metals, most often copper. Often silver covered in gold, and the silver is applied to cover bronzes, copper and other alloys. Sometimes silverware adorn niello silver marked on the figure is engraved.
The main product of corrosion of silver is silver sulfide, in the form of a thin the black film, and carbonate of copper, forming a crust of green. Less typical, but more difficult to clean, is the chloride of silver, representing gray the coating on the metal. Copper carbonate is easily removed by immersion in 5% a solution of citric or sulfuric acid. When the product seeing spots copper carbonate, you need to decide if your product is manufactured of sterling silver with a high copper content or are they just covered with a layer of silver. In the latter case, when immersion of the product in acid, you can spoil a thing by dissolving the coating. Therefore, if there is a suspicion coating, test a small area first a drop of very dilute acids.
Silver chloride is much more difficult to remove. It forms a solid film germinating inside the metal. To clean this product is by electrolysis, using as electrolyte caustic soda. After this treatment must be boiled the product in distilled water, changing the water several times, then dry at a temperature of 105°C.
In some cases, the patina is formed by chloride of zinc. If she has a beautiful shade then even desirable, especially on coins.
To remove the patina, you can use the following method. Wrap the product in aluminum foil, place in a glass jar. Add a little baking soda and pour in a jar of hot water After a while, when there is no selection bubbles, rinse the piece in water. If required, repeat the process.
If the product is not deformed very much, it can be straightened without heat. However, some thin coins will eventually become very fragile and can to break in bending. Therefore, when a strong deformation of the product should anneal.
Products made of high-grade silver maintenance-free. Alloys with low silver content is better preserved when covered with a protective varnish.
Gold and its alloys
Gold is rarely used for the manufacture of coins and products . Natural gold contains sometimes a high percentage of silver (50%). This alloy is called electrum. For practical application in gold add silver or copper, sometimes both metals. As a result, alloys, looks like a clean gold, but significantly superior to its hardness and durability. As pure gold, and most of its alloys have high corrosion resistance and being in the ground for centuries, do not change their appearance. Products and coins from gold base often covered with a patina, that is lose Shine and looks dull and gray. Thus, some gold coins Taman the untrained eye can be mistaken for lead.
Extracted from the earth products made of high-grade gold, as a rule, the same shiny, as if they were buried yesterday. Therefore, the most that should be done is to rinse them in warm water and soap with a soft toothbrush. Stuck lumps of earth is removed with a wooden toothpick. In General, ancient gold jewelry have a matte, slightly orange color that is lost in any attempt RUB the article with a piece of cloth or leather.
Gold products are easily deformed. If they are not very much bent, they can fix without heating.
When a significant deformation of the product should be heated in a flame to dark red heat, and then lower it into the water. The process should be repeated after each bend at 20-30°. Unfortunately, the roasting process causes the loss of gold color, which you can restore in dilute sulfuric acid. However noble patina to restore much more difficult.
Gold does not require any special maintenance.
Tin, lead and its alloys
Tin and lead, along with copper, gold and silver are metals, which people have used since ancient times. Products made of these metals easily manufacturing by die casting. However, they were seldom used because of their low hardness more often they were used in the form of alloys with other metals - bismuth, zinc, antimony, etc.
Articles of lead (bullets, amulets, seals) with a long presence in the soil are covered by a film of white oxide, which under normal conditions is stable. Therefore it is not necessary to remove it. However, if the object is of small details (printing) or the delicate pattern, the oxides can be removed in 5% solution of Trilon B. Oxides with products from tin and its alloys with lead removed with difficulty, accompanied corrosion products. Therefore, it is often sufficient simply to wash the item in hot water with soap and wipe with a flannel.
Author: L. V. Bulgak